Introduction to near-infrared spectroscopy
Ever wondered how is it possible that different materials can be identified using infrared light? Well, this post is for you!
Materials and (near-)infrared light
Most of the materials which can be analysed with our machine are polymers – such as polyethylene or cellulose (the main component of paper and cotton). These polymers consist of atoms arranged in long chains, typically containing atoms like carbon, hydrogen, oxygen, nitrogen and chlorine. Some chemical formulas of polymers are shown below – the brackets with a little n is a scientific notation for “repeats many times”.
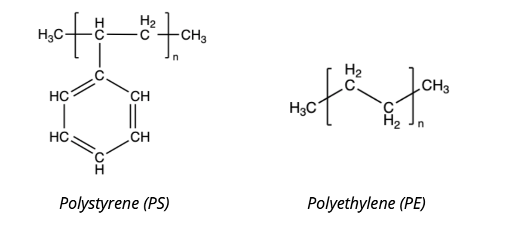
All the atoms vibrate – as if they were balls connected by springs. A typical hydrogen bonded to a carbon atom vibrates back and forth about 90 trillion times per second. This happens to be the about same frequency of vibration as that of infrared light waves. This makes the infrared light interact with the bonds between the various atoms and it is absorbed by the sample when the frequency of the atoms’ vibration matches the light’s frequency.
Measuring near-infrared spectra
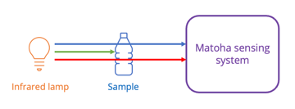
By plotting the amount of light passing through the sample as a function of wavelength, we’ll get a spectrum of the sample which serves as its unique fingerprint.
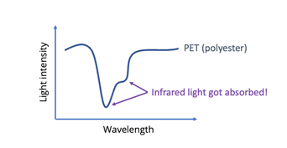
Different materials will have different spectra – and we use that to identify them!
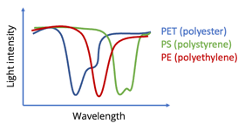
Identifying materials
As you could see in the previous section, different materials have different spectra – so how can we go from a spectrum to the material identity?Identifying blends (FabriTell-only)
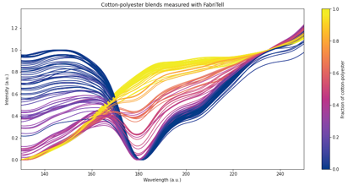
The spectra of the blends are essentially a combination of the spectra of the materials making up the blend. The graph below demonstrates this – yellow are pure cotton spectra while blue are pure polyester. The colours in between correspond to varying ratios of cotton and polyester.
--------
